Funding opportunity for SMEs to develop innovative medical manufacturing solutions using advanced inkjet printing technologies. The Open call plans to distribute €540,000 among 9 selected projects!
About About Inkjet bioAM Open Call
Inkjet bioAM is an ambitious European project designed to modernise the manufacturing of medical products and pharmaceuticals by integrating advanced inkjet printing technologies into production processes. The project responds to the pressing need for sustainable, digitised, and patient-centred healthcare solutions across Europe. By fostering smart manufacturing and accelerating the uptake of mature innovations, Inkjet bioAM aims to strengthen the resilience and competitiveness of the European biomedical sector.
The programme brings together a consortium of 15 partners, including research institutions, SMEs, multinational companies, and clusters, covering 11 EU regions.
At the core of the initiative are three pilot lines, each addressing a different aspect of biomedical manufacturing:
- Pilot Line 1:Inkjet Printing for Functionalisation of 3D Implants
- Pilot Line 2: Inkjet Printing for Functionalisation of Biosensors
- Pilot Line 3 – Inkjet Printing for Personalisation of Pharmaceutical Products
The Inkjet bioAM project will launch an open call distributing up to €540,000 among 9 selected third-party projects (3 projects developed within each pilot line). The call is dedicated to SMEs aiming to test and advance new ideas by using the technologies established in the project. Successful applicants will contribute to the value chain of their selected pilot through a co-creation process with the project partners.
Each funded project can last up to 9 months and receive up to €60,000, covering 100% of eligible costs.
You can watch a recording of their project’s online presentation here.
Success stories from previous programmes
While this is the first open call under Inkjet bioAM, it is built on proven results from prior European projects that matured the underlying technologies.
- INKplant Project (EU Horizon 2020): Developed customised 3D-printed implants for cartilage regeneration using multi-material inkjet printing. The project successfully demonstrated the feasibility of combining biodegradable inks and hydrogels to create implants tailored to individual patient anatomy, paving the way for personalised regenerative medicine.
- SMIS Project (Romanian Funding Programme): Advanced inkjet printing techniques to produce smart biosensors capable of detecting biomarkers in body fluids. This work led to functional prototypes of paper-based biosensors.
- COMET-2D Printing for Pharmaceutical Applications (National Research Initiative): Explored the feasibility of printing active pharmaceutical ingredients using inkjet technology. The project successfully manufactured test batches of pharmaceutical patches and demonstrated controlled drug release.
Why Apply to Inkjet bioAM Open Call?
Participating SMEs will gain:
- Financial Support: Up to €60,000 per project, fully covering eligible costs.
- Co-creation Opportunities: Work alongside expert research and industry partners to develop and validate innovative products.
- Access to Pilot Lines: Use cutting-edge equipment and validated processes.
- Market Readiness: Tailored support to advance innovations to market deployment.
- Visibility and Promotion: Showcase results through EU dissemination channels and industry events.
Who Can Apply?
Applicants must be companies (SMEs) and fulfil the following criteria to have the project proposal evaluated:
- Be legally established in the regions covered by the consortium (NL41, DE24, DE25, RO21, AT31, AT22, AT21, ES6), or in another less developed region of the EU or an outermost region. Eligible regions can be double-checked here.
- Be legally recognised and have to qualify for EU funding
- Demonstrate no conflict of interest with Inkjet bioAM beneficiaries
- Should not have had convictions for fraudulent behaviour, other financial irregularities, unethical or illegal business practices
- Propose projects starting at Technology Readiness Level (TRL) 6 or higher
Type of activities that can be funded
This call will fund projects that develop activities that contribute to the investment project by carrying out necessary and missing tasks, including completing specific value chains, providing groundwork for testing and validation and/or exploring new processing technologies or developing new use cases using the pilot lines.
Examples of activities by the pilots line include:
Pilot Line 1: 3D Implants Functionalisation
- Development of hydrogels (minimum TRL6) capable of enhancing chondrogenesis, delivering growth factors, and enabling cell encapsulation for bioprinting.
- Demonstration of implant applications in phantom, cadaver, or veterinary studies, optionally including clinical realisation concepts for patient-matched devices.
- Concept studies on preparing printers for GMP compliance, including air flow analysis, sterile handling, and part insertion protocols.
- Development of GMP-certifiable software systems with user-friendly interfaces.
Pilot Line 2: Biosensors Functionalisation
- Supplying materials for biosensors, such as additional receptor layers or functional coatings.
- Expertise in automating mass production and scaling of biosensor manufacturing.
- Contributions to developing other IoT sensor use cases adaptable to future pilot lines.
- Software development for user interfaces and readout applications for the existing biosensor prototypes.
Pilot Line 3: Personalised Pharmaceutical Products
- Formulation development of new printable active pharmaceutical ingredients (APIs).
- Software development supporting GMP-compliant logistics, tracking, and workflow integration.
- Equipment modifications or integration of new process modules to enhance the LP50 printer platform.
- Testing and validation of pharmaceutical patches in pharmacy or hospital environments.
How to Apply?
Proposals must be submitted electronically via the Inkjet bioAM website contract form in the following link. Submissions through any other channel will not be accepted.
The proposal must include the following mandatory elements:
- Basic information: Project title, summary, and applicant details
- Legal information: Proof of SME status and eligibility
- Project description: Addressing the proposed TRL6 technology
- Declarations: As specified in the Annexe 2 Project proposal template
H4: Important Dates
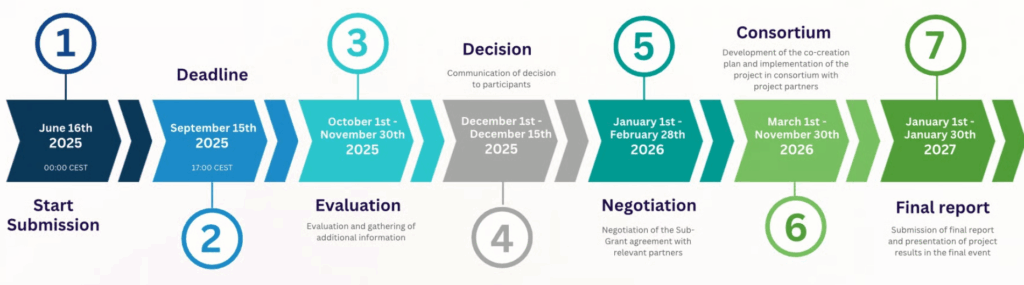
- Submission Deadline: 15/09/2025 – 17:00 (CEST)
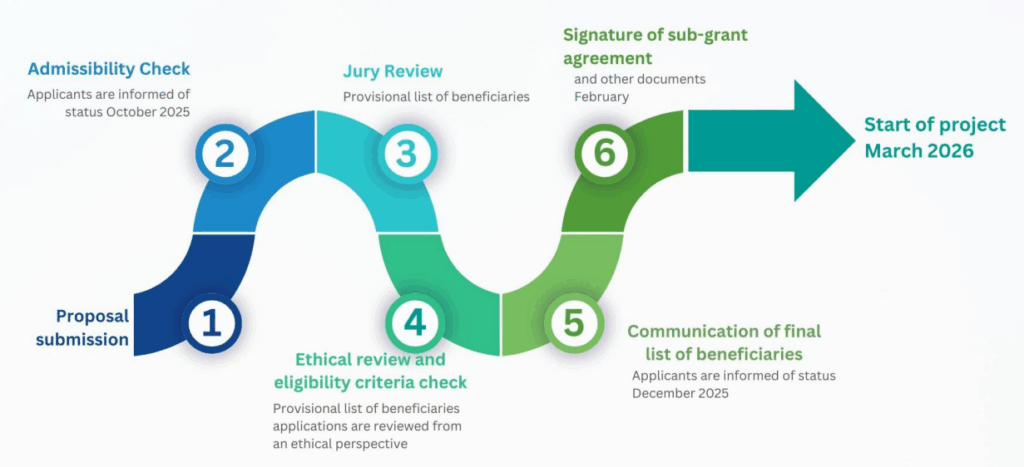
- Information on evaluation of results: December 2025
- Sub-grant agreement signature: February 2026
Project execution timeframe: March 2026
Evaluation & Selection Process
Proposals will undergo a structured evaluation process, consisting of three main phases:
- Admissibility Check: Verification of basic eligibility criteria, completeness of documentation, and compliance with submission requirements.
- Jury evaluation: The evaluation board will be composed of members of the Inkjet bioAM management board, members of the external advisory board and a representative from the partner organisations hosting each pilot line. Proposals will be scored on the following aspects, each with a maximum value of 3 points:
- Quality and innovation potential
- Contribution to the value chain of the inkjet bioAM project
- Utility of the Inkjet bioAM technologies and technical viability
- Prospective analysis (feasibility and sustainability of results)
- Additionally, SMEs from less developed or outermost regions can receive 1 bonus point
Each proposal may receive up to 13 points in total. The minimum threshold for selection is 6 points.
Ties will be resolved by prioritising proposals submitted by SMEs from less developed regions and, if needed, based on their medical relevance.
- Ethical review: the proposal shortlisted for funding will be reviewed to ensure compliance with EU ethical standards. Any projects presenting ethical issues will be asked to submit clarifications or, if necessary, may be excluded.
Final Thoughts on the Inkjet bioAM Open Call
Inkjet bioAM offers SMEs a unique opportunity to advance innovation in biomedical manufacturing by combining funding support with access to cutting-edge pilot lines and collaboration with experienced partners. This call is designed to help companies validate their technologies, demonstrate feasibility in real-world settings, and contribute to building resilient European value chains in healthcare production.
More Information
For more information:
- Project website
- Open Call details
- Support email opencall@inkjetbioam.com